The glowing gherkin
Posted on Sat 13 August 2022 in physics
DISCLAIMER
DON'T DO THIS AT HOME IF YOU DO NOT ABSOLUTELY KNOW WHAT YOU ARE DOING!
PLAYING AROUND WITH ELECTRICITY FROM A WALL-SOCKET IS LIFE-THREATENINGLY DANGEROUS!
My friend Jake McCool made a video presentation of the central aspects of this article. Check it out on youtube!
Introduction

This image was originally posted on wikipedia under a CreativeCommons-License (CC BY 3.0).
Using a stand, cables and two nails or something similar, a gherkin is connected to a wall socket via switch:
Notice, this experiment is run using alternating current (AC) as is standard in central Europe.
In practice, one should elaborate the setup a little more to prevent leak currents and make it more secure, but the important thing is to make the electric current from the wall socket go from one end of the gherkin to the other.
Observations
On powering on, the gherkin starts glowing after a short while - but only on one end. (In fact, the glow will usually be flickering a bit around one end, but stay there. In rare cases, the glow can jump from one end to the other before staying there.)
It is useful to realize the following points when searching for a qualitative explanation:
- The gherkin is glowing in yellow when the current is switched on.
- The glow usually does not cover the whole thing and is flickering. (In fact, this depends on the gherkin brand. Some gherkins can indeed be fully covered with the glow.)
- The glowing area is located around one end and tends to stay there.
Excursus: Why is the gherkin glowing, anyway?
Of course, someone might wonder, why the gerkin is glowing at all.
The glowing gherkin actually even has its own wikipedia-article, as mentioned before. It does not go into a lot of depth, but the references are quite helpful.
Therefore, the article you are reading will only cover the basic things:
- The gherkin had been placed into salted water. There is salt inside it.
- As from a chemist's point of view, salt is sodium chloride (NaCl), it contains (ionized) sodium and chlorine.
- The ionized part means, that a salt molecule is broken up into individual elements, that are not electrically neutral but overall charged. The sodium ions have a positive elementary charge.
- Furthermore, The molecules and ions are heated up by arcing due to the voltage which makes the sodium emit light. (Just like with a sodium-vapor lamp.)
Now, for the interesting part.
(Apparently) breaking symmetry
Now, why will the sodium ions (largely) go to one end of the gherkin to glow there?
After all, they are subjected to alternating current.
In order to get a qualitative understanding, we start with a gross simplification.
We replace the nails and the gherkin with a simple ideal capacitor and let the ions move freely inside it - even without influencing each other. But the movement shall only be between the poles of the capacitor, not on a "curve". With these assumptions, this problem becomes one-dimensional.
In this model, AC currents over a capacitor produce a homogenious electric field ${\mathcal E}$ that depends on time like
$${\mathcal E}(t)={\mathcal E}_0\cdot \sin{\left(\omega t +\varphi\right)},$$
where ${\mathcal E}_0$ is the amplitude, $\omega=2\pi f$ is the angular frequency and $\varphi$ is the phase at time $t_0=0$. Notice, that while the amplitude could be chosen with either sign, it will be considered positive, as is the usual approach. If at $t=0$, the field strength were negative, this would be achieved by properly choosing the phase $\varphi$.
The force $F(t)$ acting on the sodium ions (being charged with one positive elementary charge $q=1e$) becomes:
$$\begin{align} F(t)=& q{\mathcal E}(t)\\ =& q{\mathcal E}_0\sin{\left(\omega t +\varphi\right)}\\ \end{align}$$
In turn, a particular sodium ion of mass $m$ is subject to an acceleration $a(t)=\dot v(t)=\ddot x(t)$ by Newton's second law. This acceleration is:
$$\begin{align} \ddot x=& \frac{F(t)}{m}\\ = &\frac {q{\mathcal E}_0}m \sin{\left(\omega t +\varphi\right)}\\ \end{align}$$
Integration over times gives the velocity:
$$\begin{align} v(t)=&\dot x(t)\\ =&-\frac{q {\mathcal E}_0}{m\omega}\cos{\left(\omega t +\varphi\right)}+c\\ \end{align}$$
The integration constant $c$ can be calculated, if at time $t=0$ , the ion's velocity is known to be $v_0$:
$$\begin{align} v_0=& v(0)\\ =&-\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}+c,\\ \end{align}$$
thus
$$c=v_0+\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi},$$
and finally
$$\boxed{v(t)=-\frac {q {\mathcal E}_0}{m\omega}\cos{\left(\omega t+\varphi\right)}+v_0+\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}.}$$
Another integration with respect to time gives the location. (Again, $x_0$ shall be the location at time $t=0$.)
$$x(t)=-\frac {q {\mathcal E}_0}{m\omega^2}\sin{\left(\omega t+\varphi\right)}+\left(v_0+\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}\right)\cdot t + x_0+\frac {q {\mathcal E}_0}{m\omega^2}\sin{\varphi}$$
This is, indeed, the "solution" to our problem. However, we merely mention it for the sake of completeness, because it is more helpful to look at the average velocity $\langle v\rangle$ of an ion to understand the net drift in one direction.
In any case, we can have a look at the $x(t)$-function.
For the sake of simplicity, we will plot for
$$\begin{align} \varphi =& 0\\ v_0 =& 0\\ x_0 =& 0\\ \frac{q {\mathcal E}_0}{m\omega} =& 1.\\ \end{align}$$
$\omega$ can still be varied. Thus, we get the functions
$$x_{\hat\omega}(t) = -\frac 1{\hat\omega} \sin{\hat\omega t} +t.$$
Of course, this is just telling us about the general structure of the location-time-relation, but we already see a case, where $x$ is overall increasing with $t$.
Now, let us understand the effect of the phase $\varphi$. Using
$$\begin{align} \omega =& 1\\ v_0 =& 0\\ x_0 =& 0\\ \frac{q {\mathcal E}_0}{m\omega} =& 1,\\ \end{align}$$
we get the functions:
$$x_{\varphi}(t)=-\sin{\left(t+\varphi\right)}+ \cos{\varphi} \, t + \sin{\varphi}$$
There, we have it. Depending on the phase $\varphi$, an ion does a net movement into the positive or the negative direction.
Let us now get a better understanding by calculating the average velocity.
Average velocity
The average velocity can be calculated by integration over a full period $T=\frac{2\pi}\omega$ of the voltage (or the electric field, respectively), starting at an arbitrary point in time $t_R$:
$$\begin{align} \langle v\rangle= & \frac 1T \int\limits_{t_R}^{t_R+T} v(t)\, dt\\ = &\frac 1T \int\limits_{t_R}^{t_R+T} -\frac {q {\mathcal E}_0}{m\omega}\cos{\left(\omega t+\varphi\right)}+v_0+\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}\, dt\\ = &\frac 1T \underbrace{\left[ -\frac {q {\mathcal E}_0}{m\omega^2}\sin{\left(\omega t+\varphi\right)}\right]_{t_R}^{t_R+T}}_{=0}+\frac 1T \left[\left(v_0+\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}\right)t \right]_{t_R}^{t_R+T}\\ \end{align}$$
Therefore:
$$\langle v\rangle=v_0+\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}$$
Indeed, we once again obtain a non-zero average velocity, i. e. an effective movement into one direction with AC voltage. This crucially depends on the unimposing initial phase $\varphi$, that probably was not given a lot of thought in advance. Let us assume, $v_0$ is positive. (Which is just for the sake of simplicity.) Having no net drift into any direction means
$$\begin{align} \langle v\rangle=& 0\\ v_0+\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}=& 0\\ \cos{\varphi}=& -\frac{v_0}{\frac {q {\mathcal E}_0}{m\omega}}\\ =& -\frac{m\omega v_0}{q {\mathcal E}_0},\\ \end{align}$$
which is only possible, if
$$-1 \leq \frac{m\omega v_0}{q {\mathcal E}_0}\leq 1,$$
due to the cosine's boundaries.
Since $\frac{m\omega v_0}{q {\mathcal E}_0}$ is positive, by assumptions:
$$\frac{m\omega v_0}{q {\mathcal E}_0}\leq 1$$
This implies:
$$\boxed{v_0\leq \frac{q {\mathcal E}_0}{m\omega}}$$
Now, we can compute an estimate. With an effective voltage of
$$U_{eff}=230\mbox{V}$$
(as is common in central Europe), the voltage's amplitude is
$$U_0=\sqrt{2}\cdot U_{eff}\approx 325\mbox{V}.$$
If the gherkin (or really the capacitor in our simplified model) has length
$$d=4\mbox{cm},$$
we now need the permittivity of saltwater, which is soemthing like
$$\varepsilon_r\approx 32$$
to obtain
$$\begin{align} {\mathcal E}_0 = & \frac{U}{\varepsilon_r d}\\ \approx & 2.5\cdot 10^2 \frac{\mbox{V}}{\mbox{m}}.\\ \end{align}$$
Notice, that the permittivity $\varepsilon_r$ does not physically fit the model of an empty (vacuum) capacitor. We use the capacitor to approximate what is happening inside the gherkin. The permittivity is therefore just used to get a better estimate for the field strength, as it kind of factors in the matter inside the gherkin.
A sodium atom's mass is about
$$m\approx 23\mbox{u}\approx 3.82 \cdot 10^{-26} \mbox{kg}$$
and the elementary charge is
$$q\approx 1.602 \cdot 10^{-19} \mbox{C}.$$
With
$$\omega=2\pi f \approx 3.14 \cdot 10^2\frac 1{\mbox{s}}$$
we reach
$$\begin{align} v_0\leq & \frac{q {\mathcal E}_0}{m\omega}\\ =&\frac{2.5\cdot 10^2 \frac{\mbox{V}}{\mbox{m}}\cdot 1.602 \cdot 10^{-19} \mbox{C}}{3.82 \cdot 10^{-26} \mbox{kg}\cdot 3.14 \cdot 10^2\frac 1{\mbox{s}}},\\ \end{align}$$
or ultimately:
$$\boxed{v_0\leq 3.3 \cdot 10^6 \frac{\mbox{m}}{\mbox{s}}}$$
So, if we have confidence, that a sodium atom won't go from London to New York in less than two seconds, it should always be possible to find an initial phase $\varphi$ that leads to an average velocity of $0$. Thus, for smaller initial ion velocities, it is possible to find an initial phase leading to a net drift into either direction for all the ions at the same time.
Unfortunately, the phase cannot really be chosen at will, since the voltage goes through 50 cycles per second. We simply cannot "pick" the point in time we wish for to start the voltage. So, it is basically random.
Since the direction of the net drift is dominantly depending on the initial phase $\varphi$, it is in turn also "basically random".
As an ion's initial velocity $v_0$ will usually be smaller than
$$\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi},$$
by orders of magnitude, we can neglect it and approximate:
$$\langle v\rangle=\frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}$$
Now, how good is our ions-are-freely-moving-inside-an-ideal-capacitor-model?
What might be missing in this model
The obvious issue with the speed
We have calculated the average velocity to be
$$\langle v\rangle \approx \frac {q {\mathcal E}_0}{m\omega}\cos{\varphi}.$$
This depends on the initial phase $\varphi$, which is basically random. If we assume a uniform distribution for the phase, we can calculate something like an expected average velocity. However, if we only care for its magnitude and not the direction, we can limit ourselves to $\varphi\in\left[-\frac\pi 2,\frac\pi 2\right]$.
Now, we have to be careful. In terms of statistics, $\langle v\rangle$ is a function of the random variable $\varphi$, whose probability distribution can be denoted as $\psi(\varphi)=\frac 1\pi$ (since we assume a uniform distribution) for the apropriate interval.
The expected value for the average velocity is then given by:
$$E[\langle v\rangle (\varphi)] = \int \langle v\rangle (\varphi) \cdot \psi(\varphi) \, d\varphi$$
Diving into the calculations, we get:
$$\begin{align} E[\langle v\rangle (\varphi)] =& \int \langle v\rangle (\varphi) \cdot \psi(\varphi) \, d\varphi\\ =& \int\limits_{-\frac\pi 2}^{\frac\pi 2} \frac {q {\mathcal E}_0}{m\omega}\cos{\varphi} \cdot \frac 1\pi \, d\varphi\\ =& \frac {q {\mathcal E}_0}{ m\omega} \cdot \underbrace{\frac 1\pi \int\limits_{-\frac\pi 2}^{\frac\pi 2} \cos{\varphi} \, d\varphi}_{\frac 2\pi}\\ \end{align}$$
And finally,
$$E[\langle v\rangle (\varphi)] = \frac 2\pi \frac {q {\mathcal E}_0}{ m\omega} \approx 2.1 \cdot 10^6 \frac{\mbox{m}}{\mbox{s}}.$$
This implies, that on average, one would expect the ions to travel more than $2000 \mbox{km}$ into either direction within the first second of the voltage being switched on.
Ion-interactions to the rescue!
Luckily, we do not need to throw the whole explanation out of the window, just yet. So far, we completely neglected the interactions between the ions.
The sodium ions and the chlorine ions are seperated by the electric field, but since they have opposite charges, they attract each other, which will slow down the ions' drifting apart.
Coulomb's law would give the force $F_{12}$ between two charges $q_1$ and $q_2$ that are seperated by a distance $r$ to be
$$\left|F_{12}\right| =k_e \frac{\left|q_1 q_2\right|}{r^2},$$
where we limited ourselves to the one-dimensional case, again. $k_e$ is Coulomb's constant, which is approximately:
$$k_e \approx 8.988 \cdot 10^9 \frac{\mbox{N}\mbox{m}^2}{\mbox{C}^2}$$
Consider a sodium ion and a chlorine ion which both are separated by half the gherkin's length $d$. They are both charged with one elementary charge with opposite signs, which we will ignore. (This simply tells us, that the ions will attract each other.)
In that case, we can estimate the force:
$$\begin{align} \left|F_{12}\right| = & k_e \frac{\left|q_1 q_2\right|}{r^2}\\ = & 8.988 \cdot 10^9 \frac{\mbox{N}\mbox{m}^2}{\mbox{C}^2} \frac{\left( 1.602 \cdot 10^{-19} \mbox{C}\right)^2}{\left( 2 \cdot 10^{-2} \mbox{m}\right)^2}\\ \approx & 8.988 \cdot 10^9 \frac{2.566 \cdot 10^{-38} }{4 \cdot 10^{-4}} \mbox{N}\\ \approx & 5.8 \cdot 10^{-25} \mbox{N}\\ \end{align}$$
Now, what kind of acceleration would that produce for the sodium?
Well, the sodium ions' mass was $m\approx 3.82 \cdot 10^{-26} \mbox{kg}$ and thus, we get an acceleration of
$$\left|a_{12}\right| = \frac{\left|F_{12}\right|}m \approx 1.5 \cdot 10 \frac{\mbox{m}}{\mbox{s}^2},$$
which is way to small to combat the acceleration due to the field.
But what happens when the ions are closer together, as they are in the beginning?
Well, if the distance decreases by a factor of $10$, the force increases by a factor of $100$, because of the $r^2$-term in the denominator. So, it makes sense, that in the beginning (i. e. when turning on the voltage), the forces resisting the field are much stronger than after some separation of the ions had happened.
Furthermore, moving all the sodium ions to one end of the gherkin will shrink their distance and increase the forces between them, which are repulsive. That would be another effect slowing down the movements of the ions as induced by the electric field.
Gherkin structure to the rescue!
Then, there is the structure of the gherkin itself.
From a physicist's point of view, this is a highly complicated system, as the gherkin's cells are made of a number of molecules, that are not simple, themselves. This could certainly account for some friction forces.
It is definitely conceivable, that this limits the movement of the ions in a considerable manner. (If this was not the case, inertia should make the ions leave the gherkin quickly and we would not see any glow for long.)
Non-homogenious electric field
We assumed the electric field to be homogenious for the sake of simplicity in our calculations. But in the end, what effect will the deviation from homogeneity have? In any case, the homogenious field component that accelerates the ions will weaken. (In the homogeneous case, the whole field points into or completely opposite to the direction of movement, which means that this is the configuration that will get most of the field's energy into the acceleration towards the gherkins end. Any deviation from the homogeneity will result in weaker acceleration.)
Why is the chlorine not glowing at the other end?
This one is at least potentially easy.
A guy named Neill Tucker has rendered an emission spectrum graph for chlorine and released it to wikipedia:
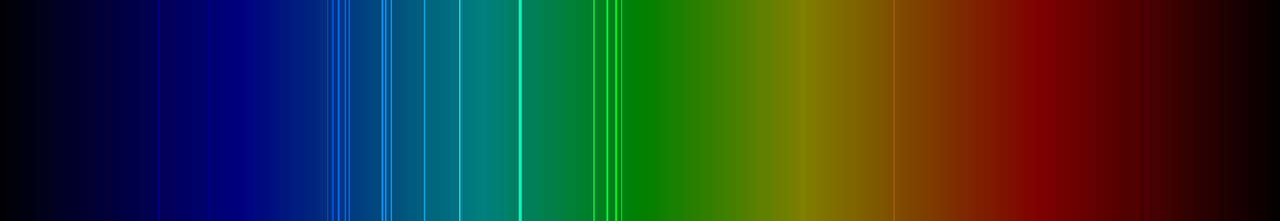
As we can see, the most dominant spectral frequencies are in the green and blue wavelengths, meaning they correspond to higher energy.
Since the chlorine ions are also heavier than the sodium ions, it seems plausible that the chlorine might simply require more energy.
A possible experiment to check this would be to just increase the voltage.
Effects that were ignored
Aside from the stuff just mentioned, there are other things we did not dive into. (And we won't do that now, either. It just felt negligent to not mention them at all.)
-
Asymmetry of the gherkin itself
The gherkin is not symmetrical. One end has a shaft where the gherkin was connected to the rest of the plant and the other one basically layed around, freely. This might lead to systematic differences in movability of ions.
-
Limits of Newtonian physics
We have not considered any quantum mechanics, which is currently the best theory for dealing with small objects like ions. For small objects, Newtonian physics can only be considered an approximation. Furthermore, we are potentially talking about speeds on the order of magnitude of $10^6 \frac{\mbox{m}}{\mbox{s}}$, which is about $1\%$ the speed of light. This is probably a speed range that does not yet require relativistic calculations, but if any value in our estimation were to change one order of magnitude, this might change, as well.
-
Other elements in the gherkin and chemistry
There are, of course, other elements and molecules present inside the gherkin, who might lead to additional interactions. If nothing else, there would be some sort of friction.
But surely, it is possible, that some chemical interactions occur. Especially since the sodium and the chlorine are ionized. That might produce new molecules with different properties.
Wrap up
Ultimately, this system is way to complicated to do a full physical description. Indeed, this is, why we started with that gross oversimplification in the first place.
This is quite a common problem when doing science. Real-world problems frequently evade to be captured by a complete model considering everything important. Usually, one can only hope to enclose the most important effects when writing down a model to describe, what is happening.
Here, there are two take-aways. On one hand, this article proposes a model to understand one particular effect of the glowing gherkin demonstration, namely the glow appearing only on one end of the gherkin.
More generally, the reader should have learned that a sinusoidal force does not neccessarily imply a sinusoidal motion, which is probably not the most intuitive fact.


